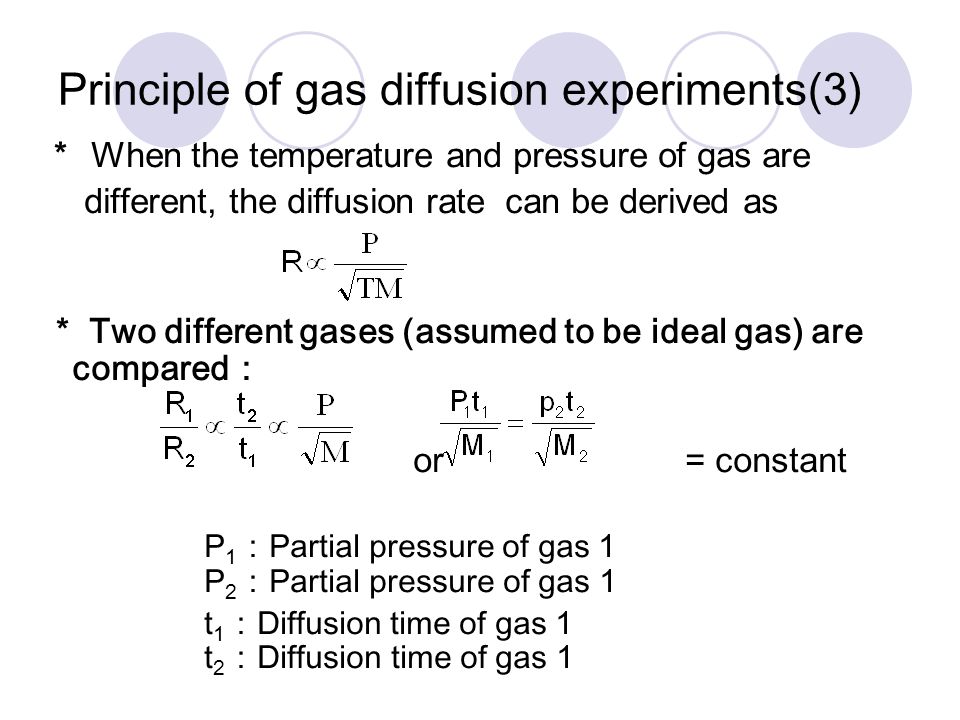
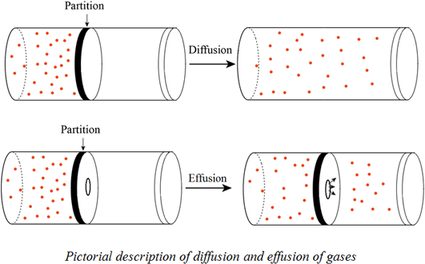
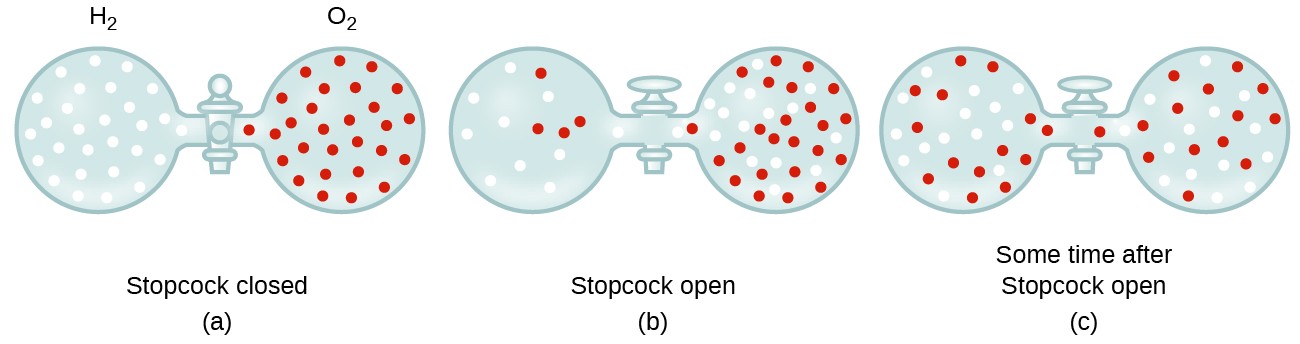
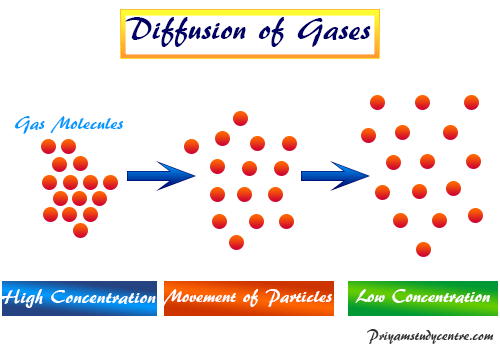
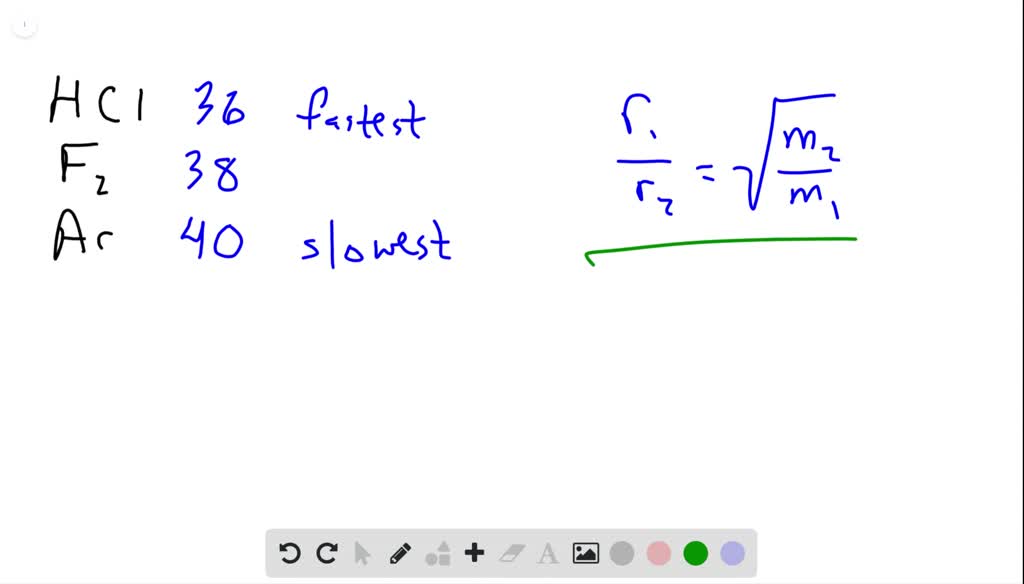
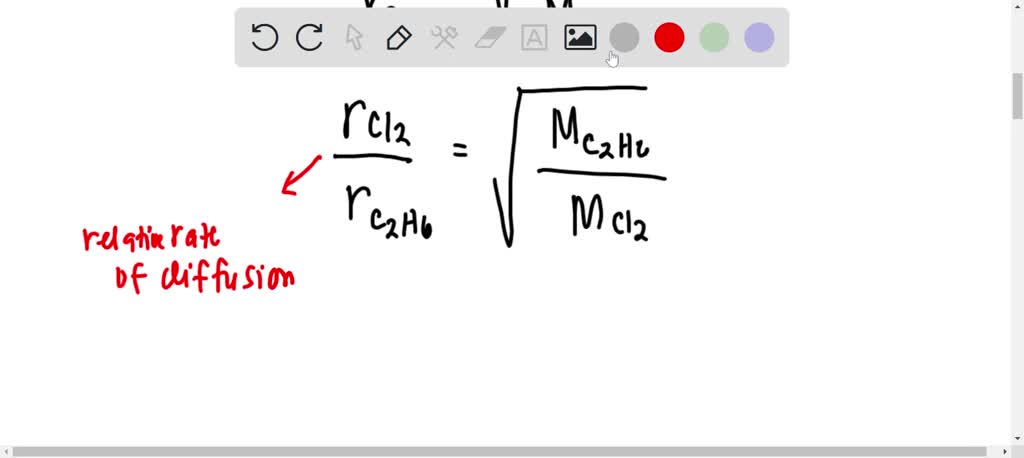
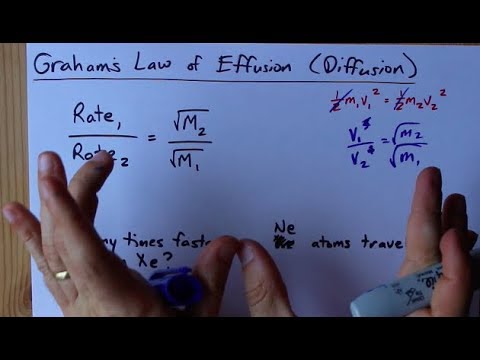
63.Rate of diffusion of gas X is twice that of gas Y if molecular mass of Y is 64 then the molecular mass of X will be

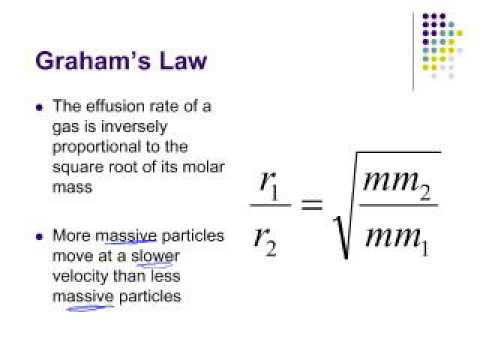
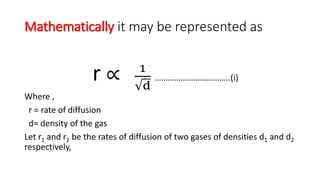
Rate of diffusion of gas is :directly proportional to its molecular massdirectly proportional to square of its molecular massinversely proportional to the square root of its moleculer massdirectly proportional to its vapour

![ANSWERED] SolveLancer Test The rate of diffusion of two gases X Y are - Kunduz ANSWERED] SolveLancer Test The rate of diffusion of two gases X Y are - Kunduz](https://media.kunduz.com/media/sug-question-candidate/20210625230951982656-1884508.jpg)